Dropping Bottle Control Procedure
A sample of a freshly prepared Black Oxide production bath should always be taken as a control solution prior to running any parts through the bath. If a sample was not taken, a laboratory prepared solution at the same concentration may be used as the control solution. Titration of this “new”” solution will provide the figure for D1.
- Transfer a 5 ml sample of the production bath into a 125 ml Erlenmeyer flask.
- Dilute with water to the 50 ml mark.
- Add 2 ml 6N (1:1) Hydrochloric Acid to the flask.
- Add 4 ml of the 15% by weight Potassium Iodide solution.
- Add 2 ml of Starch solution. The solution will become a dark blue to almost black color.
- Add the 0.5N Sodium Thiosulfate solution, from the dropping bottle – drop by drop – counting the drops while swirling the flask.
- The end point is marked by a sudden change in color from dark black to light brown.
Note: Upon standing, the light brown color will turn dark again, but additional Sodium Thiosulfate solution should not be added. The first end point is correct.
- Calculate the amount of concentrate to be added as follows:
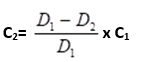
C2= Black Oxide concentrate in gallons to be added to the bath
D1= Number of drops of Sodium Thiosulfate used to titrate the new production bath.
D2=Number of drops of Sodium Thiosulfate used to titrate the used production bath.
C1 =Volume of Black Oxide concentrate in gallons used to make up the original new” bath.
